Modernize Testing
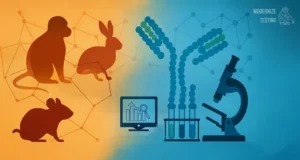
Animal testing is outmoded because, unlike human biology-based test methods, animals are unreliable predictors of human response, delaying treatments and cures to patients and driving up drug costs. The FDA must adapt its regulations to keep up with the law.

The Issue
More than two years ago, Congress passed the FDA Modernization Act 2.0 and President Biden signed the bill into law. It was without question the most important policy in the nation’s history addressing animal testing. The bill eliminated an 84-year-old animal-testing mandate for every new drug in development, whether a drug for cancer, pain, Alzheimer’s, or any other affliction of the human body.
This new law promised a reboot of our national policies on drug development, sparing the lives of millions of animals a year, saving drug developers billions because they could now use more reliable and less expensive screening methods, and delivering better lifesaving and life-enhancing treatments and cures for tens of millions of patients in need. But Biden’s FDA failed to act.
With recent announcements from FDA, EPA, and NIH to wind down animal testing, however, the new Administration is promising to shed the inhumane, inefficient, and archaic model of animal testing and to substitute a new era characterized by human-relevant science and personalized medicine.
The Solution
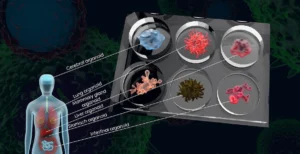
Congress must compel the FDA to update its regulations so drug-developers can take advantage of modern scientific methods and end the horrors suffered by animals in their labs. We continue to push for the implementation of the new law through the FDA Modernization Act 3.0. The legislation requires the FDA to publish a final rule to implement the FDA Modernization Act 2.0 and establish clear guidelines for non-animal test methods that can better predict drug safety and efficacy, and speed the time to market for new treatments and cures.
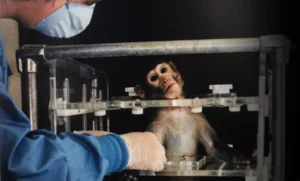
The NIH must also wind down the work of the seven regional primate research and breeding facilities. The average cost of a primate used in drug testing now exceeds $50,000 and fails to deliver reliable information to the scientists involved in these protocols.
The centers have failed at their most basic level of providing “resources” and finding cures for human ailments for six decades, yet the taxpayer funding continues, despite the promise of groundbreaking vaccines and cures. After six decades of taxpayer funding and hundreds of thousands of non-human primates falling victim to unnecessary animal testing, we still have a 90-95% failure rate in human clinical trials largely because animals are not appropriate surrogates for the human condition.
Actions to Take
Tell your legislators
Other Campaigns
View our Webinars
Watch Our Videos
Read more about our campaign
Circular photo credit:
Beagle: Jo-Anne McArthur / Animal Equality / We Animals Media, Capuchin monkey: Jo-Anne McArthur / NEAVS / We Animals Media